Biosurfactants: The Superheroes of Sustainable Chemistry
Welcome aboard the voyage into the fascinating realm of biosurfactants! In this article, we’re diving deep into the world of these microscopic marvels – nature’s own guardians of the surface tension galaxy.
While the term might sound like a mouthful, biosurfactants play a crucial role in various industries, from skincare to paints and coatings. But fear not, as we embark on this educational journey, we promise to infuse it with a healthy dose of fun!
What Are Biosurfactants?
Let’s kick things off with a bit of science, shall we? Biosurfactants are amphiphilic compounds produced by microorganisms, plants or animals.
Amphiphilic, you ask? It means they have both hydrophilic (water-loving) and hydrophobic (water-repelling) properties. This unique combination allows them to reduce surface tension, making it easier for substances to mix.
Think of biosurfactants as tiny molecular superheroes, breaking down barriers between substances that normally wouldn’t mingle.
How Do Biosurfactants Work?
Biosurfactants work by reducing the surface tension between two immiscible phases, such as water and oil, or between a liquid and a solid surface. They achieve this through their unique molecular structure, which consists of both hydrophilic and hydrophobic regions.
The amphiphilic nature of these molecules allows them to adsorb at the interface between different phases, effectively lowering the surface tension and facilitating the formation and stabilization of emulsions or foams. Here’s a step-by-step explanation of how biosurfactants work:
-
Adsorption
When biosurfactant molecules encounter the interface between two immiscible phases, such as water and oil, they adsorb at the interface with their hydrophilic heads oriented towards the aqueous phase and their hydrophobic tails facing away from the water.
-
Surface Tension Reduction
By adsorbing at the interface, biosurfactants disrupt the cohesive forces between molecules at the surface, effectively reducing the surface tension. This reduction in surface tension allows the immiscible phases to mix more easily and form stable emulsions or foams.
-
Emulsion Formation
In the case of water and oil, biosurfactants help to create and stabilize emulsions by surrounding oil droplets with a layer of surfactant molecules. This layer prevents the oil droplets from coalescing and separating from the aqueous phase, thereby maintaining the stability of the emulsion.
-
Foam Stabilization
Biosurfactants also play a role in stabilizing foams by reducing the surface tension of the liquid-air interface within the foam bubbles. This reduction in surface tension helps to prevent the coalescence of bubbles and maintain the structural integrity of the foam.
-
Interfacial Activity
In addition to their role in emulsion and foam formation, biosurfactants can also exhibit interfacial activity at solid-liquid interfaces. They can modify the surface properties of solid materials, such as increasing wetting or spreading on solid surfaces, which can have applications in cleaning, agriculture and enhanced oil recovery.
Overall, biosurfactants work by adsorbing at interfaces between immiscible phases, reducing surface tension and facilitating the formation and stabilization of emulsions, foams and modified solid surfaces. Their unique properties make them valuable in various industrial, environmental and personal care applications.
How Are Biosurfactants Classified?
Biosurfactants can be classified based on their molecular weight, chemical composition, microbial origin and molecular structure. Here are the primary classification criteria:
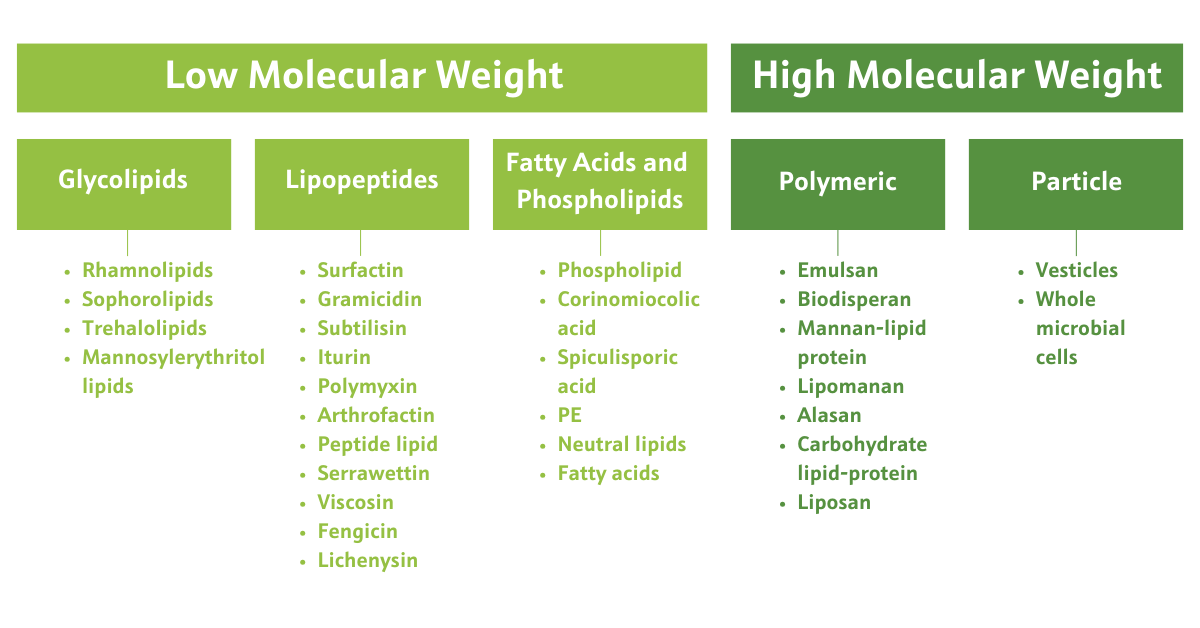
Molecular Weight
Biosurfactants can be classified by low and high molecular weights:
- Low Molecular Weight:
- This group includes biosurfactants with relatively small molecular weights, typically ranging from around 200 to 1000 Daltons.
- Examples of low molecular weight biosurfactants include lipopeptides, such as surfactin produced by Bacillus species, and glycolipids, such as rhamnolipids and sophorolipids.
- These biosurfactants are composed of shorter carbon chain lengths and simpler structures, often consisting of a hydrophilic head group (e.g., peptide or sugar moiety) attached to a hydrophobic tail (e.g., fatty acid).
- Low molecular weight biosurfactants are known for their surface-active properties, including reducing surface tension and forming stable emulsions.
- High Molecular Weight:
- This category encompasses biosurfactants with larger molecular weights, typically exceeding 1000 Daltons.
- Examples of high molecular weight biosurfactants include lipopolysaccharides (LPS) produced by certain bacteria and polysaccharide-based biosurfactants.
- High molecular weight biosurfactants are characterized by complex structures, often composed of long polysaccharide chains with hydrophobic and hydrophilic regions.
- These biosurfactants may exhibit more diverse functionalities beyond surface activity, including immunomodulatory effects and biofilm regulation.
Fig. 1 Biosurfactants classified by molecular size and chemical composition.
Chemical Composition
Biosurfactants can be classified based on their chemical composition, which includes:
- Glycolipids: Glycolipids consist of a sugar (glyco-) moiety attached to a lipid chain. Examples include sophorolipids and trehalolipids.
- Lipopeptides: Lipopeptides consist of a peptide (protein) chain linked to a lipid (fatty acid) chain. Examples include surfactin and iturin.
- Phospholipids: Phospholipids are composed of a phosphate group, glycerol backbone and fatty acid chains. Phospholipids are commonly found in cell membranes.
- Polymeric: Polymeric biosurfactants are a class of biosurfactants composed of long-chain polymers produced by microorganisms. Unlike low molecular weight biosurfactants such as glycolipids or lipopeptides, polymeric biosurfactants consist of macromolecules with repeating monomeric units linked together through covalent bonds.
- Particle: Particulate biosurfactants play a pivotal role in the partitioning of extracellular membrane vesicles, leading to the formation of a microemulsion that significantly impacts alkane uptake in microbial cells.
Microbial Origin
Biosurfactants are produced by various microorganisms, including bacteria, fungi, yeast and algae. Each microbial species may produce different types, contributing to the diversity within this classification.
Glycolipids
| Microorganism | Application | Biosurfactant Class |
|---|---|---|
| P. aeruginosa and P. putida | Bioremediation | Rhamnolipids |
| P. chlororaphis | Biocontrol agent | Rhamnolipids |
| Bacillus subtilis | Antifungal agent | Rhamnolipids |
| Renibacterium salmoninarum | Bioremediation | Rhamnolipids |
| Candida bombicola and C. apicola | Emulsifier, MEOR, alkane dissimilation | Sophorolipids |
| Rhodococcus spp. | Bioremediation | Trehalose lipids |
| Tsukamurella sp. and Arthrobacter sp. | Antimicrobial agent | Trehalose lipids |
| Candida antartica | Neuroreceptor antagonist, antimicrobial agent | Mannosylerythritol lipids |
| Kurtzmanomyces sp | Biomedical application | Mannosylerythritol lipids |
Lipopeptides
| Microorganism | Application | Biosurfactant Class |
|---|---|---|
| Bacillus subtilis | Antimicrobial agent, biomedical application | Surfactin |
| B. licheniformis | Hemolytic and chelating agent | Lichenysin |
Fig. 2 Glycolipid and lipopeptide biosurfactants classified by microbial origin.
Molecular Structure
Biosurfactants can also be classified based on their molecular structure, such as their hydrophilic and hydrophobic components. This classification helps in understanding their surfactant properties and applications.
Biosurfactant Families
Within each classification, biosurfactants can further be grouped into families based on similarities in structure, function and biosynthesis pathways. For example, the rhamnolipid family includes different variants of rhamnolipids produced by various microbial species.
These classification schemes help researchers and industries better understand the properties and potential applications of different types of biosurfactants. Additionally, they aid in the selection of biosurfactants for specific industrial or environmental applications based on their unique characteristics.

Case Study: Biosurfactant Production in Five Steps
Biosurfactant manufacturing involves several steps that encompass microbial fermentation, extraction, purification and formulation. Here’s a general overview of the biosurfactant manufacturing process:
-
Microbial Fermentation
Biosurfactants are typically produced by microorganisms such as bacteria, yeast and fungi. The first step in manufacturing involves cultivating these microorganisms in a suitable fermentation medium.
The fermentation medium contains carbon and nitrogen sources, along with other nutrients necessary for microbial growth and biosurfactant production. The fermentation process is carried out under controlled conditions of temperature, pH and oxygen supply to optimize biosurfactant yield.
-
Biosurfactant Production
During fermentation, the microorganisms produce biosurfactants as secondary metabolites. Biosurfactant production occurs during the growth phase of the microorganisms, and the biosurfactants are typically secreted into the fermentation broth. The biosurfactants reduce the surface tension of the medium, forming micelles and stabilizing emulsions.
-
Extraction
Once biosurfactant production reaches its peak, the fermentation broth is harvested, and the biosurfactants are extracted from the broth. Various extraction methods can be employed depending on the nature of the biosurfactants and the microorganisms used. Common extraction techniques include solvent extraction, precipitation and centrifugation.
-
Purification
After extraction, the crude biosurfactant extract undergoes purification to remove impurities and concentrate the biosurfactants. Purification techniques may include chromatography, filtration and precipitation. These methods help isolate the biosurfactants from other components present in the fermentation broth, resulting in a more refined product.
-
Formulation
Once purified, the biosurfactants may undergo formulation to prepare them for specific applications.
Formulation may involve blending the biosurfactants with other ingredients to enhance stability, functionality or compatibility with different products or processes.
The formulated biosurfactants can then be packaged and distributed for use in various industries such as agriculture, bioremediation, cosmetics and pharmaceuticals.
Throughout the manufacturing process, quality control measures are implemented to ensure the consistency, purity and effectiveness of the biosurfactants.
Quality control measures may include analytical testing, microbial assays, and product characterization. Additionally, efforts are made to optimize the manufacturing process to increase yield, reduce production costs and minimize environmental impact.

What Are “Novel” Biosurfactants?
A novel biosurfactant refers to newly discovered or synthesized molecules that possess unique characteristics, properties or applications compared to previously known biosurfactants.
These novel molecules may be identified through screening of microorganisms, genetic engineering techniques or other synthesis methods. These biosurfactants often exhibit diverse chemical structures and surface-active properties and functionalities, making them of interest for various industrial, environmental and personal care applications.
Characteristics of novel biosurfactants may include:
- Unusual Chemical Structures: They may have chemical structures that differ from traditional surfactants, leading to unique surface-active properties and interactions with other molecules.
- Enhanced Stability: Some may exhibit increased stability under extreme conditions such as temperature, pH and salinity, making them suitable for use in harsh environments.
- Improved Biodegradability: They may be designed or discovered to have enhanced biodegradability, reducing environmental impact and promoting sustainability compared to conventional surfactants.
- Tailored Functionality: Through genetic engineering or chemical modification, they can be tailored to possess specific functionalities such as antimicrobial activity, emulsification, foam stabilization or drug delivery.
- Novel Applications: They may also open up new avenues for applications in industries such as agriculture, bioremediation, cosmetics, pharmaceuticals, food processing and enhanced oil recovery.
Overall, novel biosurfactants represent an exciting area of research and development, offering potential solutions to challenges in various fields while also advancing our understanding of microbial physiology, molecular biology and biotechnology.
Six Examples of Novel Biosurfactants
Several examples of novel biosurfactants have been discovered or synthesized in recent history, each with unique properties and potential applications. Some examples include:
Sophorolipids
Sophorolipids are glycolipid biosurfactants produced by yeast species such as Candida bombicola. They have excellent surface-active properties, biodegradability and low toxicity, making them suitable for applications in cleaning products, agriculture and petroleum industries.
Trehalolipids
Trehalolipids are glycolipids produced by bacteria such as Rhodococcus and Mycobacterium species. They exhibit surface-active properties, emulsification capabilities and low cytotoxicity, making them promising candidates for applications in cosmetics, pharmaceuticals and bioremediation.
Mannosylerythritol Lipids (MELs)
MELs are glycolipids produced by yeast species such as Candida antarctica and Pseudozyma species. They exhibit excellent surface-active properties, low toxicity and biodegradability, making them suitable for applications in cosmetics, pharmaceuticals and environmental remediation.
Tensioactives
Tensioactives are produced by the fungus Fusarium oxysporum. They exhibit surface-active properties, antifungal activity and potential applications in agriculture, cosmetics and pharmaceuticals.
Rhamnolipids
Rhamnolipids are glycolipids produced by various bacterial species, including Pseudomonas aeruginosa. They have attracted attention for their surface-active properties, antimicrobial activity and potential applications in bioremediation, enhanced oil recovery and medical treatments.
Cyclic Lipopeptides
Cyclic lipopeptides such as surfactin, iturin and fengycin are produced by bacteria belonging to the Bacillus genus. They possess strong surface-active properties, antimicrobial activity and potential applications in agriculture, bioremediation and pharmaceuticals.
These examples represent just a few of the many novel biosurfactants that have been discovered or synthesized in recent years. Continued research and development in this field is likely to uncover even more diverse biosurfactants with unique properties and applications.
What Is The Difference Between A Surfactant And A Biosurfactant?
While chemical surfactants undoubtedly demonstrate efficiency and versatility, it’s imperative to recognize the significant drawbacks linked to their widespread utilization.
The environmental ramifications of chemical surfactants, particularly those sourced from non-renewable petrochemical origins, cannot be underestimated. Their production and disposal contribute to pollution, posing enduring threats to ecosystems.
Furthermore, the limited biodegradability of specific chemical surfactants raises apprehensions regarding their persistence in the environment, potentially causing ecological harm.
Health considerations also demand attention, as certain chemical surfactants may harbor risks, especially when utilized in high concentrations or in products directly contacting the skin. These concerns align with an escalating awareness of the necessity for more sustainable and eco-friendly alternatives amidst mounting environmental challenges.
Chemical surfactants have played a pivotal role across various industries, but their overall impact necessitates a reevaluation of their usage. The ongoing pursuit of greener and safer alternatives underscores a collective acknowledgment that the environmental and health concerns associated with chemical surfactants merit earnest consideration.
Raw Materials
Biosurfactants are typically produced using renewable resources such as plant oils, agricultural wastes or microbial biomass. These raw materials often have a lower carbon footprint compared to the fossil fuel-derived raw materials used in chemical surfactant production.
Chemical surfactants are often derived from petrochemicals, which are fossil fuel-based raw materials. The extraction and processing of these raw materials contribute to carbon emissions throughout their lifecycle.
Structure
Biosurfactants have diverse chemical structures and can vary widely depending on the producing organism. They may include lipids, peptides, glycolipids, phospholipids and other complex molecules. Examples of biosurfactants include rhamnolipids, sophorolipids, lipopeptides and glycolipids.
Synthetic surfactants often have a simple chemical structure consisting of a hydrophilic head and a hydrophobic tail. Common types include alkyl sulfates, alkylbenzene sulfonates and alkylphenol ethoxylates.
Production Process
Biosurfactants are often produced through microbial fermentation, which can be carried out under mild conditions without the need for high temperatures or pressures. This fermentation process may require less energy compared to the energy-intensive processes used in chemical surfactant production.
Chemical surfactant production typically involves energy-intensive processes such as petroleum refining, chemical synthesis and purification. These processes can result in significant carbon emissions due to energy consumption and chemical reactions.
Biodegradability
Biosurfactants are biodegradable compounds, meaning they break down naturally in the environment over time. This characteristic reduces the potential for long-term carbon emissions associated with their disposal compared to non-biodegradable chemical surfactants.
Many chemical surfactants are non-biodegradable or have limited biodegradability, leading to long-term environmental impacts and potential carbon emissions associated with their persistence in the environment.
Waste Generation
Biosurfactant production processes typically generate less waste compared to chemical surfactant production processes. Byproducts of biosurfactant fermentation can often be utilized or recycled, minimizing overall waste generation and associated carbon emissions.
Chemical surfactant production processes often generate large quantities of waste and byproducts, including hazardous chemicals and pollutants. Disposal of these wastes may contribute to carbon emissions through transportation, treatment, and landfilling.
Overall, while specific data may vary depending on factors such as the specific biosurfactant or chemical surfactant being produced, as well as the production methods used, biosurfactant production generally tends to have a lower carbon footprint compared to chemical surfactant production.
The lower carbon footprint of biosurfactants is primarily due to the renewable nature of raw materials, milder production processes, biodegradability and reduced waste generation.
How Can Biosurfactants Improve Formulations?
Novel biosurfactant members of the glycolipid class, like sophorolipids, offer formulators a better choice when designing profitable, high-performing and sustainable products.
Locus Ingredients’ line of Amphi® sophorolipids includes blendable lactonic and linear options that are highly efficacious for industrial markets such as :
- Agricultural Adjuvants
- CASE (Coatings, Adhesives, Sealants and Elastomers)
- I&I (Industrial and Institutional) Cleaning
- Metalworking Fluids
- Printing Ink
- Pulp and Paper
- Textiles and Leather
- Water Treatment
With the growing availability of chemical surfactant alternatives like biosurfactants, the answer is clear. Reach out to our technical service department today, and let us help you get started!
READ MORE: Examining The Versatility Of Eco-Friendly Sophorolipids
What Are Sophorolipids?
Sophorolipids are a specific type of biosurfactant with unique characteristics and applications. They are a type of glycolipid produced by certain yeast species, most notably Candida bombicola and Starmerella bombicola. These compounds are composed of a hydrophilic head group, typically a sophorose sugar molecule, and a hydrophobic fatty acid tail.
Due to their environmentally friendly nature and versatile functions, sophorolipid biosurfactants find applications in a variety of industries. Their role as emulsifiers, wetting agents, dispersants and more makes them valuable in processes ranging from cleansing and degreasing to dispersing pigments in paint.