
Coalescing Agents Transformed: Unleashing the Potential of Sophorolipid Biosurfactants
What Are Coalescing Agents?
Coalescing agents, crucial in applications like paint formulation, play a pivotal role in enhancing film formation and coating properties. They facilitate the merging of small droplets in a liquid medium, ensuring a seamless and cohesive end result.
These surface-active agents or surfactants also play an important role in enhancing film formation—a process critical to the performance and durability of paints. As paint is applied to a surface, it initially consists of small, dispersed droplets. These droplets need to seamlessly merge and fuse together to form a continuous and uniform film. Coalescing surfactants act as the guiding force, bringing dispersed droplets closer together, encouraging them to harmonize, and ultimately facilitating their fusion.
The end goal is to achieve a paint film that appears seamless, without visible gaps or irregularities. Surfactants with coalescing abilities contribute to the cohesion of the film, ensuring that it not only looks aesthetically pleasing but also functions effectively as a protective and decorative layer.
In essence, coalescing agents are the organizers of the paint formulation process, assembling droplets like pieces of a puzzle. Now, let’s explore how sophorolipids, with their unique characteristics, step into this role, reshaping the landscape of coalescing agents in a sustainable and effective manner.
Three-Step Process Of Coalescence
Now let’s try to visualize this process to better understand coalescence. In The Journal of Petroleum Technology, a three-step process is described for oil in water coalescence.
- Two droplets converge at a proximity ranging from 10 to 100 microns, prompting deformation of their approaching faces and the formation of a disc-shaped film between them. As the film undergoes drainage, surface forces, including electrostatic repulsion and van der Waals attraction, gain prominence.
- The liquid film gradually reduces to a critical thickness of 0.01 to 0.1 micron.
- Ultimately, the thin film ruptures, culminating in the coalescence of the droplets.
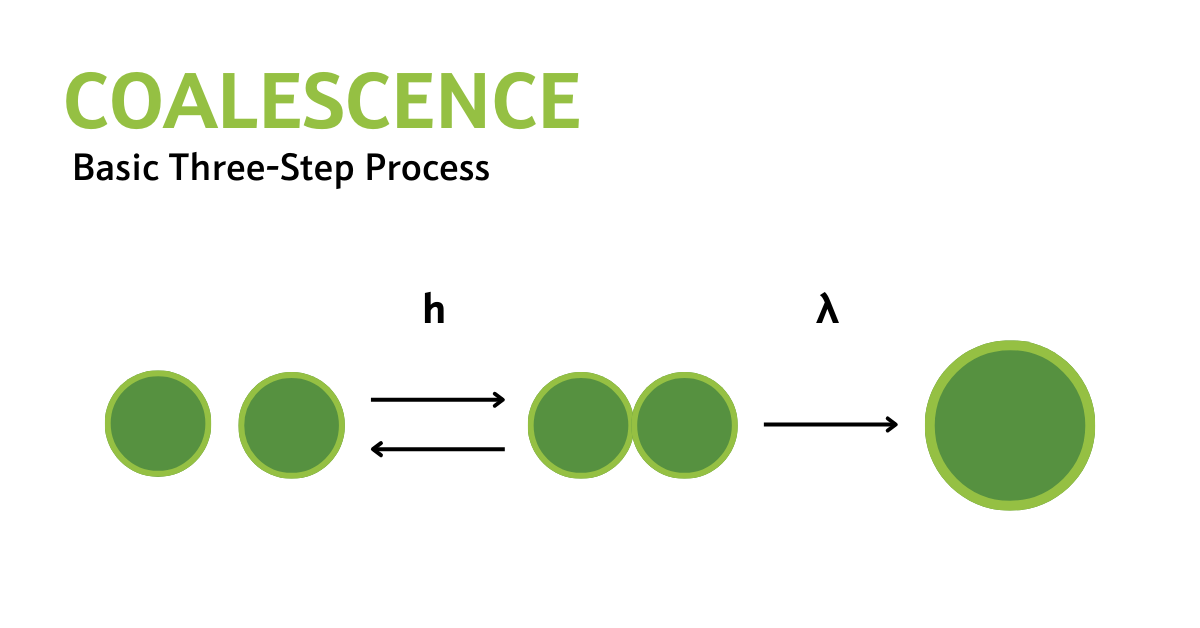
Fig. 1—Visualization depicting the coalescence and separation of dispersed droplets. “h” represents the collision rate for the formation of a doublet, while “λ” signifies the coalescence rate of the doublet. Source: Narsimhan 2004.
The rate-limiting step for coalescence can be any of the three mentioned steps. By conceptualizing coalescence as a three-step process, it becomes clear how factors like droplet collision rates, oil droplet size, oil/water interfacial tension, and oil droplet film properties influence the growth of droplet size.
Sophorolipids At A Glance
Biosurfactants are naturally occurring surface-active molecules produced by microorganisms such as bacteria, yeast, and fungi. Unlike chemical surfactants synthesized from petrochemicals, biosurfactants are derived from renewable resources and exhibit a wide range of unique properties. These molecules typically consist of both hydrophobic (water-repelling) and hydrophilic (water-attracting) components, allowing them to lower the surface tension between liquids and solids.
Biosurfactants find application in various industries, including agriculture, pharmaceuticals, food and environmental remediation. They contribute to processes such as coalescing, emulsification, foaming and dispersion, making them valuable in areas like enhanced paints and coatings, metalworking fluids, agricultural adjuvants and the formulation of eco-friendly cleaning products. The sustainable and biodegradable nature of biosurfactants makes them an attractive alternative to traditional surfactants, aligning with the increasing global emphasis on environmentally friendly solutions.
At the heart of the green biosurfactant revolution are sophorolipids—a glycolipid produced by yeast species, notably Candida bombicola and Starmerella bombicola. Their molecular structure, with a hydrophilic head group and a hydrophobic fatty acid tail, gives them a unique edge as coalescing surfactants.
Derived from the fermentation process of yeast, sophorolipids represent a bio-based alternative to traditional, often petrochemical-derived, coalescing surfactants. This eco-friendly origin aligns with the growing demand for sustainable and renewable solutions in various industries.
At the core of sophorolipids’ effectiveness as coalescing agents lies their unique molecular architecture. Picture it as a sophisticated key perfectly crafted to unlock the potential of cohesive film formation. Sophorolipids consist of a hydrophilic, sophorose sugar molecule head group and a hydrophobic, lipid fatty acid tail, a duality that positions them as exceptional agents in bridging disparate elements.
This dual nature enables sophorolipids to perform a special function at the interface between immiscible liquids, such as oil and water. The hydrophilic head group is drawn to water, while the hydrophobic tail seeks refuge in oil. This balancing act reduces the surface tension between the two phases, creating an environment conducive to the merging of droplets—an essential step in coalescence.
What sets these eco-friendly molecules apart is not just their green credentials but their versatility. In the area of coalescing agents, this versatility is a game-changer. Sophorolipids seamlessly adapt to different formulations and applications, showcasing their effectiveness not only in paint but across many varied industries.

Paint Formulation With Sophorolipids
In the field of paint formulation, sophorolipids demonstrate unique characteristics. Their ability to promote coalescence leads to improved film formation, enhancing coating properties. Unlike traditional coalescing surfactants, sophorolipids can enable the creation of volatile organic compound (VOC)-free and environmentally friendly paint formulations.
Promoting Coalescence for Enhanced Film Formation
Sophorolipids play an important role in the coalescence process, a critical step in the formulation of high-quality paints. Sophorolipids promote the merging of dispersed particles, this results in improved film formation, where the paint transforms from a collection of individual droplets into a continuous, seamless layer on surfaces.
Enhancing Coating Properties
The impact of sophorolipids extends beyond just film formation. Their presence elevates coating properties, contributing to a final product that not only looks aesthetically pleasing but also boasts enhanced durability and functionality. Paint formulations with sophorolipids showcase a superior ability to adhere to surfaces, resist wear and tear and withstand the test of time.
VOC-Free and Environmentally Friendly Formulations
One of the defining features of sophorolipids in paint formulation is their role in steering formulations toward a sustainable path. Unlike traditional coalescing surfactants that may contribute to VOC emissions, sophorolipids pave the way for VOC-free formulations. This shift aligns with the global push for environmentally friendly practices in the paint industry, where reducing VOCs is a key initiative.
Beyond Green Chemistry
Sophorolipids not only embrace the principles of green chemistry but redefine them. Their incorporation into paint formulations signifies a departure from conventional practices, signaling a commitment to a cleaner and greener future. The use of sophorolipids resonates with environmentally conscious consumers seeking products that align with their values.
Case Study: Polymer Particles And Film Formation In Latex Paints
In latex paint formulations, coalescence plays a crucial role in achieving the desired finish and durability of the painted surface. Latex paint consists of polymer particles, water and various additives. During the application of latex paint, the water evaporates, and the polymer particles come into contact with each other. The coalescence process then takes place, contributing to the formation of a continuous and cohesive film.
Here’s a simplified breakdown of how coalescence works in latex paint:
- Coating Applied: Latex paint initially contains dispersed polymer particles in an aqueous medium. When the paint is applied to a surface, the water in the formulation starts to evaporate.
- Particle Contact: As the water evaporates, the polymer particles approach each other and make contact. At this stage, the particles are still discrete and not fully fused.
- Coalescence Initiation: The coalescence process begins when the polymer particles experience a critical temperature known as the glass transition temperature (Tg). At this temperature, the polymer particles start to soften and become more malleable. With continued heating or exposure to ambient temperatures, the softened polymer particles fuse together. This fusion results in the formation of a continuous and smooth film on the painted surface.
- Cohesive Film: The coalesced film creates a uniform and cohesive layer, providing the painted surface with its final appearance and protective properties. The film ensures that the paint adheres well to the substrate and delivers the desired finish, whether it’s a matte, satin, or glossy appearance.
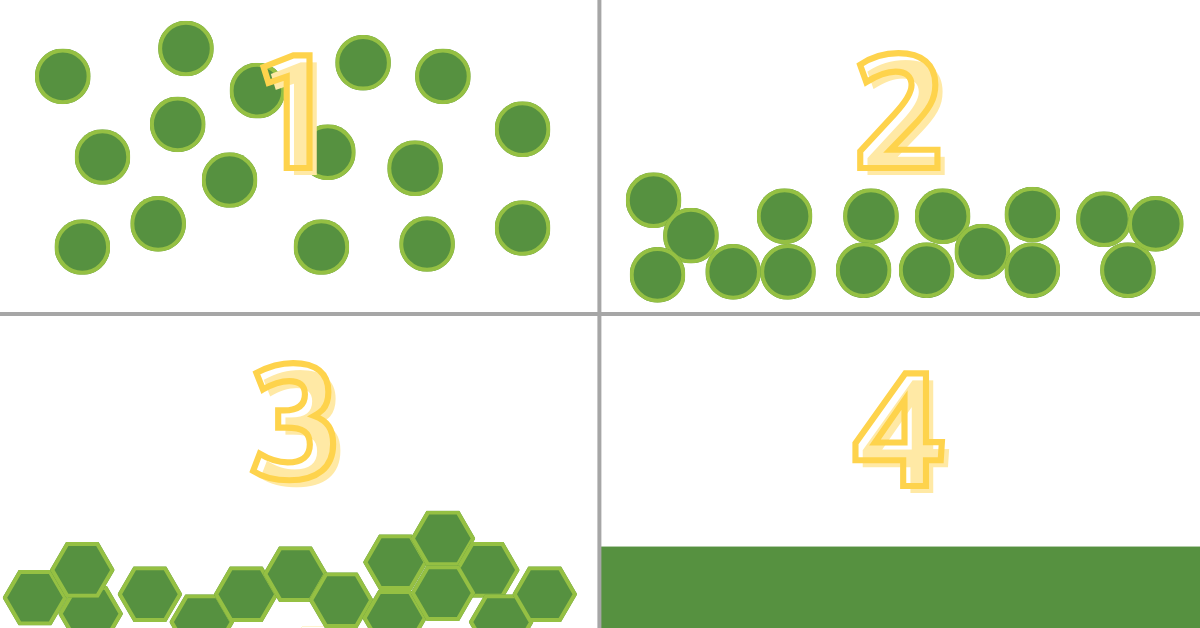
Fig. 2—Illustrates the stepwise progression of the latex film formation process. At Stage 1, the system begins as a colloidal suspension of latex particles. Progressing to Stage 2, the particles densely pack together, and capillary forces induce deformation. Stage 3 showcases the particles adopting a dodecahedral (3D honeycomb) structure, achieved when the temperature (T) surpasses the Minimum Film Formation Temperature (MFFT). Finally, Stage 4 marks the culmination of a homogeneous film, where particles seamlessly merge into a uniform structure. This phase occurs when the temperature exceeds the Tg.
Factors such as temperature, humidity and the specific formulation of latex paint can influence the coalescence process. Proper conditions and additives are often incorporated to facilitate an efficient coalescence, ensuring the formation of a durable and aesthetically pleasing paint finish.

Environmental Benefits Of Sophorolipids In CASE Applications
Sophorolipids, being derived from natural sources, carry a minimal environmental footprint. Their use as coalescing agents aligns with the growing demand for sustainable and eco-friendly alternatives in various industries.
- Renewable Sourcing – Derived from the fermentation of yeast species, notably Candida bombicola and Starmerella bombicola, these glycolipids utilize readily available and replenishable raw materials. This sustainable sourcing approach ensures that the production of these molecules does not contribute to resource depletion or disrupt delicate ecosystems. Amphi® sophorolipids from Locus Ingredients are free of palm oil, eliminating growing concerns related to rainforest deforestation and labor issues. They are also produced with a flexible, non-GMO feedstock oil so as not to compete with food chain resources.
- Eco-Friendly Production Processes – The process from yeast fermentation to sophorolipid creation is inherently eco-friendly. The production processes are designed to minimize energy consumption and environmental impact. Compared to traditional coalescing surfactants that may rely on energy-intensive manufacturing methods, Locus Ingredients’ Amphi® ingredients stand out for their efficient and sustainable production.
- Biodegradability – The biodegradable nature of sophorolipids ensures that they seamlessly integrate into natural processes, leaving no lasting impact on ecosystems or water sources. This contrasts sharply with certain synthetic alternatives that might persist in the environment, contributing to long-term pollution concerns. Amphi® ingredients are readily biodegradable, which means that at least 60% has already biodegraded in the first six days of release into the environment.
- Circular Economy:
- Reduced Carbon Footprint – The carbon footprint associated with sophorolipids is inherently lower compared to some conventional coalescing agents. Their bio-based origin means that the carbon released during their lifecycle is part of the natural carbon cycle. This reduction in carbon footprint aligns with global efforts to combat climate change and promotes a shift towards sustainable alternatives in the chemical industry.
- End-of-Life Considerations – As formulations evolve, so do considerations for end-of-life scenarios. Sophorolipids, being biodegradable, reduce concerns about persistent pollutants in waste streams. This eco-friendly characteristic aligns with circular economy principles, where ingredients are designed to return to the environment without causing harm, contributing to a more sustainable and closed-loop system.
- Meeting Consumer Demands – The growing demand for sustainable and eco-friendly products is a driving force shaping industries. By choosing Amphi® ingredients as coalescing surfactants, manufacturers respond to consumer preferences for products that not only deliver high performance but also align with environmental values. This proactive approach positions sophorolipids as key contributors to meeting the evolving expectations of environmentally conscious consumers.
In the grand scheme of formulation ingredients, sophorolipids not only perform admirably as coalescing agents but also leave behind a positive environmental legacy. Their journey from renewable sourcing to eco-friendly production processes reflects a commitment to a future where sustainability and effectiveness coexist harmoniously.

Challenges And Future Trends
While sophorolipids present a promising green alternative, it’s essential to address challenges and considerations associated with their use as coalescing agents. Understanding these factors is crucial for informed decision-making.
Cost Considerations
The environmental benefits of sophorolipids are clear, but the economic aspect requires careful consideration. Sophorolipids, being a bio-based and naturally sourced ingredient, have historically come with a higher production cost compared to some synthetic counterparts. Manufacturers must weigh the environmental advantages against the potential economic implications and assess the overall impact on product pricing.
However, Locus Ingredients has developed a novel, modular production process that has a low carbon footprint and gives formulators access to rapidly scalable and customizable ingredient options to replace a broad range of synthetic surfactants. These cost-effective solutions eliminate the “green premium” and other barriers that prevented adoption of sophorolipids in the past.
Formulation Compatibility
The versatility of sophorolipids is a strength, but it also introduces considerations regarding formulation compatibility. Certain formulations may require specific characteristics from coalescing agents, and thorough testing is necessary to ensure that sophorolipids seamlessly integrate without compromising the performance or stability of the end product.
Locus Ingredients’ formulation lab has a wealth of ready-to-use formulations that can speed the development of new products. And while re-formulation may be necessary as sophorolipids are usually not a one-to-one replacement for synthetic surfactants, they do demonstrate excellent co-surfactancy, often times replacing more than one ingredient, enabling cleaner product labels with their versatile functionality.
Availability and Regulatory Compliance
Navigating the complex regulatory landscape is a consideration that cannot be overlooked. While sophorolipids often align with sustainability goals, ensuring compliance with industry regulations and standards is essential. Manufacturers need to stay informed about any regulatory changes or requirements associated with the use of sophorolipids in various applications.
Locus Ingredients’ parent company, Locus Fermentation Solutions (Locus FS), is the first U.S.-based company to receive Toxic Substances Control Act (TSCA) for national production. The U.S. Environmental Protection Agency (EPA) has approved Locus FS’ TSCA Pre-Manufacture Notifications (PMNs) for its fermentation-produced sophorolipids across a multitude of applications. This includes usage in both industrial and consumer products and formulations. Not only can formulators and manufacturers domestically source sophorolipids from Locus Ingredients, but the central location of the production facilities in Solon, Ohio make it fast and reasonable priced to ship sophorolipids anywhere in the U.S.
Why Choose Amphi® Ingredients?
Selecting Amphi® sophorolipids as coalescing agents offers a myriad of advantages, making them a preferred choice in various applications. First and foremost, Amphi® ingredients possess exceptional amphiphilic properties, meaning they can interact effectively with both hydrophobic and hydrophilic substances. This unique characteristic is crucial in coalescence, where the merging of dispersed particles is facilitated.
Additionally, Amphi® ingredients are derived from renewable microbial sources, aligning with the growing demand for sustainable and environmentally friendly ingredients. Their natural origin contributes to reduced ecological impact, a factor increasingly prioritized in today’s formulations.
The versatility of Amphi® ingredients extends to their ability to stabilize emulsions and enhance the formation of homogeneous films, improving the overall quality and performance of products. This makes them valuable not only as coalescing agents but also in various applications such as in the formulation of paints, coatings, sealants and elastomers.
Finally, Amphi® ingredients possess a host of highly desirable, eco-friendly certifications and qualities:
- USDA certified for containing 100% biobased content
- Readily biodegradable
- Non-GMO
- Palm oil free
- Free of 1,4-dioaxane, ethylene oxide and other Proposition 65 prohibited dangerous chemicals
- Enables VOC-free paint formulations
- Made in the USA
As we conclude, the message is resounding: sophorolipids like Amphi® aren’t just additives; they’re catalysts for a formulation revolution. They exemplify the point where sustainability and functionality converge, creating a space for businesses to not only meet environmental expectations but exceed them.

READ MORE: Examining The Versatility Of Eco-Friendly Sophorolipid Biosurfactants
Key Functions and Applications
Due to their environmentally friendly nature and multifunctionality, sophorolipid biosurfactants find applications in a wide range of industries.



