Clash of the Colloids: Emulsifiers vs. Dispersants Explained
Emulsification, the process of dispersing immiscible liquids, is a crucial function in numerous industries. Bio-based emulsifiers, like sophorolipid biosurfactants that are renowned for their eco-friendly nature and versatile applications, emerge as key players in this arena.
This article delves into the multifaceted role of sophorolipids as bio-based emulsifiers, discussing their significance across diverse sectors and shedding light on their potential to revolutionize formulations.
How Does Emulsification Work?
Emulsification is the process of dispersing one immiscible liquid (the dispersed phase) into another liquid (the continuous phase) to form a stable emulsion. This process involves the use of emulsifiers, which are surfactant molecules that lower the interfacial tension between the two liquids and stabilize the resulting mixture.
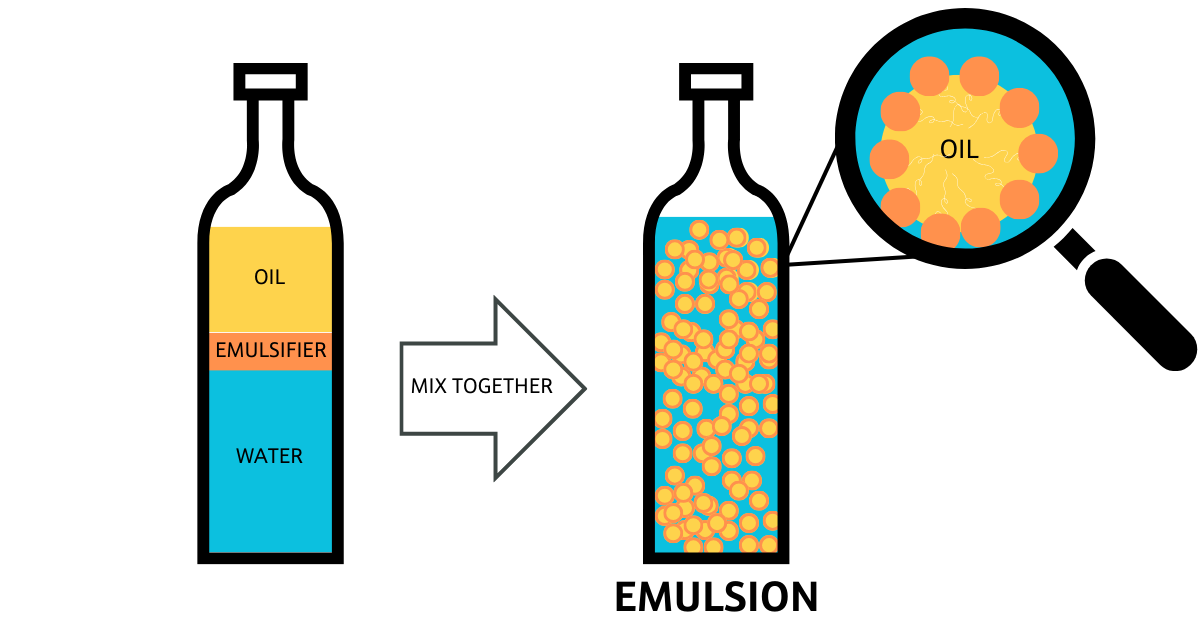
Figure 1. Emulsification example where oil is the dispersed phase and water is the continuous phase. When mixed together including an emulsifying surfactant, they create an oil-in-water (O/W) emulsion.
Emulsification in Four Steps
- Interfacial Tension Reduction: The first step in emulsification is to reduce the interfacial tension between the dispersed phase and the continuous phase. Interfacial tension is the force that exists at the boundary between two immiscible liquids and tends to minimize the contact area between them. Emulsifiers work by adsorbing onto the interface between the two liquids, disrupting the cohesive forces at the interface, and reducing interfacial tension.
- Dispersion of Dispersed Phase: Once the interfacial tension is lowered, the dispersed phase (typically oil or hydrophobic liquids) can be dispersed into the continuous phase (usually water or hydrophilic liquids). The emulsifiers form a layer around the dispersed phase droplets, preventing them from coalescing and merging back into larger oil droplets. This dispersion process is facilitated by mixing or agitation, which helps distribute the dispersed phase evenly throughout the continuous phase.
- Stabilization of Emulsion: Emulsifiers play a crucial role in stabilizing the emulsion by forming a protective layer around the dispersed phase droplets. This layer, known as the interfacial film or monolayer, acts as a barrier that prevents the dispersed phase droplets from coming into direct contact with each other and coalescing. The emulsifiers orient themselves at the oil-water interface, with their hydrophilic (water-attracting) heads facing the continuous phase and their hydrophobic (oil-attracting) tails facing the dispersed phase, creating a stable emulsion.
- Maintenance of Emulsion Stability: Emulsifiers not only prevent coalescence of the dispersed phase droplets but also provide additional mechanisms to maintain emulsion stability. These mechanisms may include steric hindrance, electrostatic repulsion and particle or droplet charge stabilization. The emulsifiers’ structure and composition, as well as the properties of the dispersed and continuous phases, influence the stability of the emulsion over time.
Overall, emulsification is a complex process that involves the reduction of interfacial tension, dispersion of the dispersed phase and stabilization of the resulting emulsion by emulsifiers.
By effectively lowering interfacial tension and forming a protective layer around dispersed phase droplets, emulsifiers enable the creation of stable emulsions with diverse applications in various industries, including cleaning, cosmetics and coatings.
What is a Colloid?
A colloid is a type of mixture where one substance is dispersed evenly throughout another. The particles in a colloid are larger than those found in a solution but smaller than those found in a suspension.
Colloids can exist in various states, including liquid, solid and gas, and are characterized by their ability to scatter light. Examples of colloids include fog (a liquid dispersed in air), milk (fat particles dispersed in water) and gelatin (solid particles dispersed in water).
Are Dispersions and Emulsifications Colloids?
Yes, both emulsions and dispersions are types of colloids.
- An emulsion is a type of colloid where one liquid is dispersed in another liquid. For example, mayonnaise is an emulsion where oil droplets are dispersed in water.
- A dispersion is also a type of colloid where solid particles are dispersed in a liquid medium. For example, paint is a dispersion where pigment particles are dispersed in a solvent.
In both cases, the dispersed phase (oil or solid particles) is dispersed evenly throughout the continuous phase (water or liquid medium), forming a stable colloidal system.
How are Dispersions and Emulsifications Different?
Dispersion and emulsification are both processes that involve the mixing of immiscible substances, but they differ in the nature of the substances being mixed and the final result. Here are the key differences between dispersion and emulsification:
| Dispersion | Emulsification | |
|---|---|---|
| Definition | Dispersion is a general term for the process of evenly distributing or dispersing solid particles in a liquid or gas medium. It can involve the dispersion of solids in liquids (suspensions) or gases, and it can also refer to the dispersion of liquids in other liquids or gases. | Emulsification specifically refers to the process of dispersing immiscible liquids (usually oil and water) to form an emulsion. Emulsions are colloidal systems where one liquid is dispersed in the form of small droplets within another liquid. |
| Components Involved | It can involve the dispersion of solids, liquids or gases in a different medium. For example, dispersing pigment particles in paint or gas bubbles in a liquid. | It specifically involves the dispersion of two immiscible liquids, typically oil and water, to form an emulsion. |
| Resulting Mixture | The result of dispersion can be a suspension (solid particles in a liquid or gas), an aerosol (small particles dispersed in a gas) or the dispersion of one liquid in another. | The result is an emulsion is a stable mixture of two immiscible liquids. Emulsions can be oil-in-water (O/W) or water-in-oil (W/O), depending on the continuous phase. |
| Process Mechanism | Dispersion can be achieved through various mechanisms, such as mechanical stirring, milling or the use of dispersing agents that reduce inter-particle attraction. | Emulsification typically involves the use of emulsifying agents or surfactants that stabilize the interface between the immiscible liquids, preventing their separation. |
| Examples | Mixing paint pigments in a liquid medium, creating a suspension of solid particles in a liquid. | Making salad dressing by emulsifying oil and vinegar, creating a stable mixture of oil droplets dispersed in water. |
In summary, while both dispersion and emulsification involve mixing immiscible substances, dispersion is a broader term that encompasses various types of mixtures, including the dispersion of solids, liquids or gases. Emulsification, on the other hand, specifically refers to the creation of stable emulsions by dispersing two immiscible liquids.
What is the Tyndall Effect?
The Tyndall effect, named after the 19th-century physicist John Tyndall, is a phenomenon observed when light is scattered or dispersed by colloidal particles or very fine particles suspended in a transparent medium, such as a gas or a liquid.
When a beam of light passes through a colloidal dispersion, the individual particles in the dispersion scatter the light in all directions. This scattered light makes the path of the beam visible, resulting in a visible cone of light that appears when the beam is viewed from the side.
The intensity of the scattered light depends on factors such as the size, shape and concentration of the particles, as well as the wavelength of the incident light.
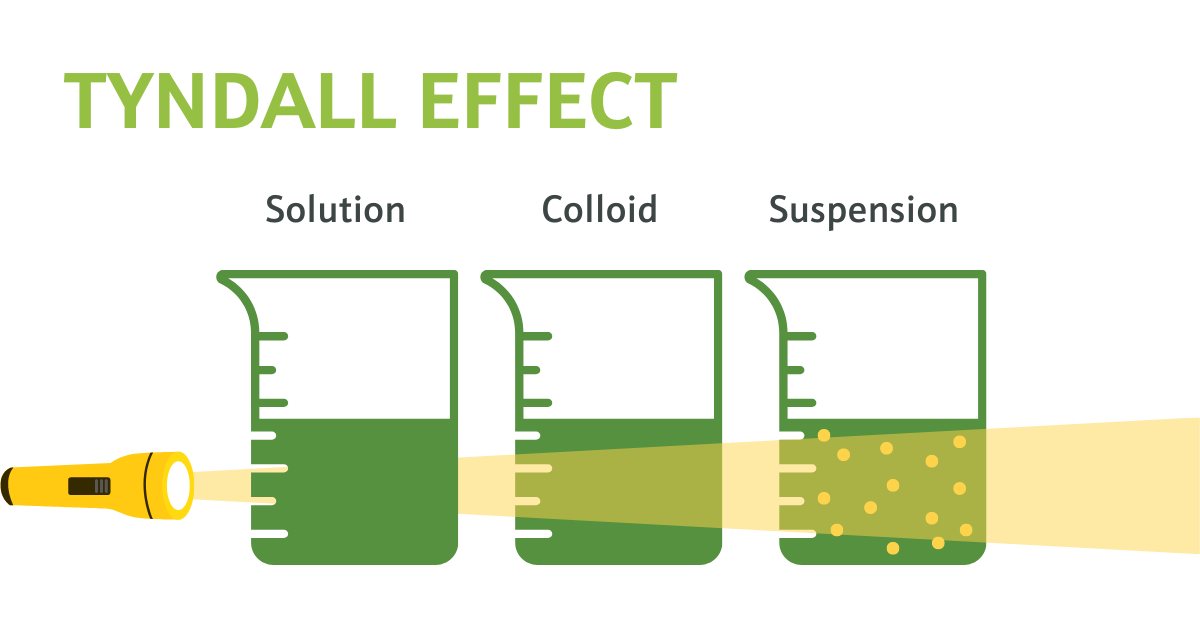
Figure 2. Visual demonstration of the Tyndall Effect: Light scattering by particles in a colloid and a suspension showcase colloidal dispersion evidenced by the visible beams of light. The suspension, on the other hand, shows no beam of light as there are no particles present to reflect it.
The Tyndall effect is commonly observed in everyday situations. For example, when sunlight passes through a dusty room, the dust particles scatter the light, making the beam of light visible.
Similarly, the Tyndall effect is responsible for the blue color of the sky, as the gas molecules and fine particles in the Earth’s atmosphere scatter sunlight, particularly the shorter blue wavelengths, in all directions.
In scientific applications, the Tyndall effect can be used to determine the presence of colloidal particles in a solution or to study the properties of colloidal dispersions. It has various practical applications, including in the fields of chemistry, biology and materials science.
How Are Coalescing Agents Related to Emulsification?
Coalescing agents and emulsification are related processes that occur in different contexts but share some underlying principles.
- Coalescing agents are additives used in formulations, particularly in paints and coatings, to promote the fusion or merging of individual droplets of dispersed phase (such as latex particles) into larger droplets. This process is known as coalescence.
- Coalescing agents reduce the barrier to droplet coalescence, allowing them to come into close contact and merge, ultimately forming a continuous film.
- Emulsification, on the other hand, involves the creation of stable emulsions by dispersing one immiscible liquid phase (such as oil) into another immiscible liquid phase (such as water), with the aid of emulsifiers or surfactants.
- Emulsifiers reduce the interfacial tension between the two phases, allowing them to mix and form stable droplets or globules within the continuous phase.
Relationship between Coalescing Agents and Emulsification in Latex Paint Formulations
While coalescing agents and emulsification may seem like opposing processes, they are actually complementary in certain formulations, such as latex paints.
In latex paint formulations, emulsions of polymer particles (latex) are dispersed in water. During the application of the paint, the water evaporates, and the latex particles coalesce to form a continuous film.
Coalescing agents facilitate the process by promoting the merging of latex particles, leading to improved film formation and coating properties.
Therefore, coalescing agents indirectly influence emulsification by aiding in the stability and performance of emulsions, particularly in systems where the dispersed phase needs to coalesce to form a continuous film.
In summary, while coalescing agents and emulsification occur in different contexts, they are interconnected in certain formulations, where coalescing agents facilitate the fusion of dispersed phase droplets to form a continuous film, ultimately impacting the stability and performance of emulsions.
What are Bio-Based Emulsifiers?
Bio-based emulsifiers are surfactants derived from renewable sources such as plants, microbes or other biological materials. They serve as crucial ingredients in various formulations, including cleaning products, paints, coatings and personal care products.
Bio-based emulsifiers are needed for several reasons:
- Environmental Sustainability: Bio-based emulsifiers offer a sustainable alternative to petroleum-based surfactants, which are derived from finite fossil fuel resources. By utilizing renewable feedstocks, bio-based emulsifiers reduce dependency on non-renewable resources and contribute to a more sustainable manufacturing process.
- Reduced Environmental Impact: The production and use of bio-based emulsifiers typically result in lower carbon emissions and reduced environmental footprint compared to their synthetic counterparts. They often exhibit better biodegradability and lower toxicity, minimizing adverse effects on ecosystems and human health.
- Regulatory Compliance: With increasing regulatory scrutiny on chemical ingredients and their environmental impact, the use of bio-based emulsifiers helps companies comply with stringent regulations and meet sustainability targets. Many regulatory agencies prioritize the use of renewable and environmentally friendly ingredients in consumer products.
- Consumer Demand: There is a growing consumer preference for eco-friendly and sustainable products across various industries. Bio-based emulsifiers align with consumer values of environmental responsibility and health consciousness, driving demand for products formulated with these ingredients.
- Performance Benefits: Bio-based emulsifiers can offer performance benefits comparable to or even superior to traditional surfactants. They can enhance stability, dispersion and solubilization properties in formulations, leading to improved product performance and efficacy.
Overall, bio-based emulsifiers play a vital role in advancing green chemistry initiatives and promoting sustainable practices in various industries. They offer a win-win solution by providing effective functional properties while minimizing environmental impact and meeting the demands of eco-conscious consumers.
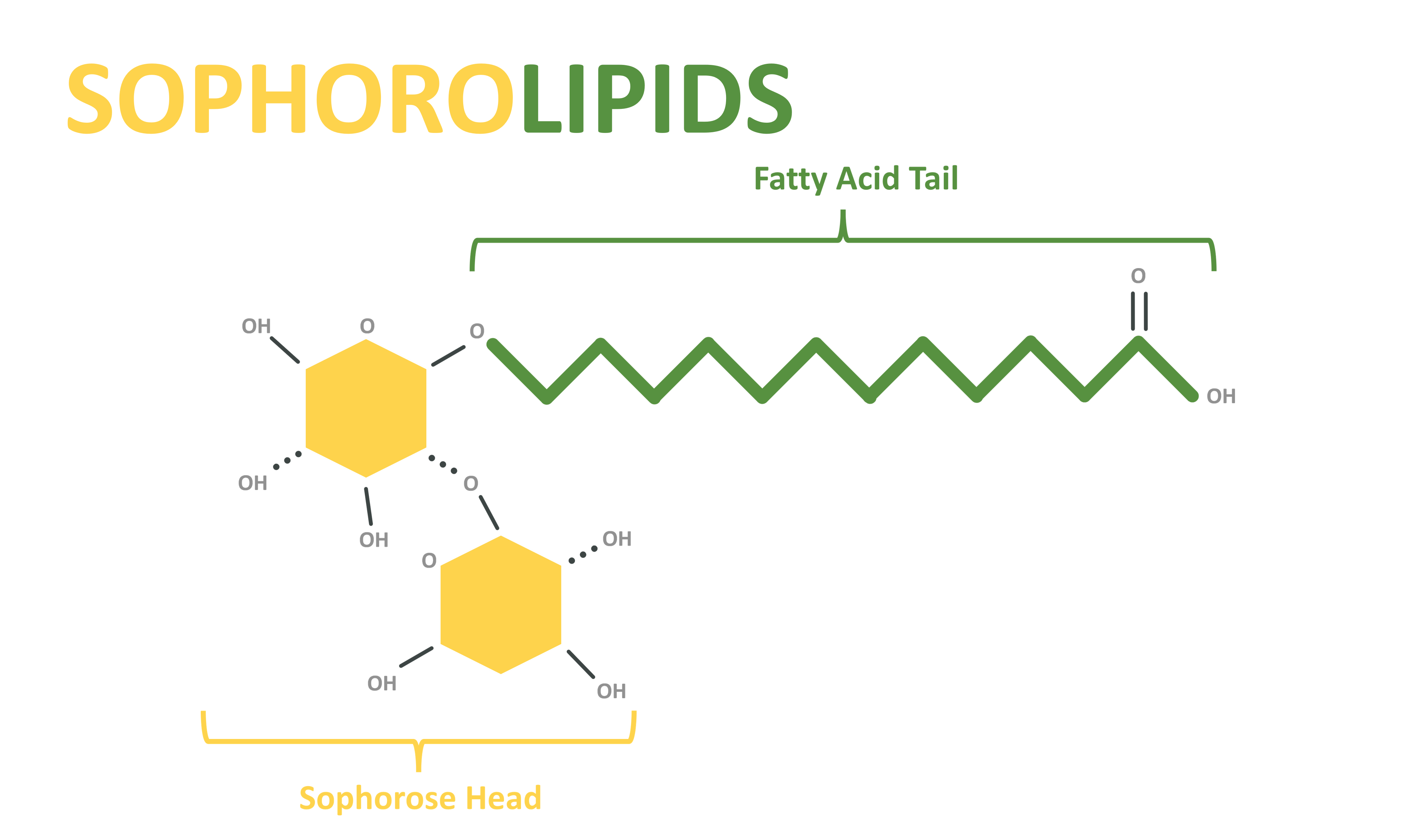
Figure 3. Acidic, or linear, sophorolipids structure.
Sophorolipid Biosurfactants as Bio-Based Emulsifiers
Sophorolipid biosurfactants represent a promising category of bio-based emulsifiers derived from microbial fermentation of renewable feedstocks, such as vegetable oils or glucose. These unique molecules possess exceptional surface-active properties, making them highly effective as emulsifiers in a wide range of applications.
Key Characteristics and Benefits of Sophorolipid Biosurfactants as Bio-Based Emulsifiers
Renewable Sourcing
Sophorolipids are produced through microbial fermentation, utilizing renewable feedstocks as substrates. This sustainable production process reduces reliance on finite fossil resources and contributes to a more environmentally friendly supply chain.
Biodegradability
Sophorolipid bio-based emulsifiers are readily biodegradable, meaning they can be broken down by natural processes into non-toxic compounds. This property makes them environmentally benign and reduces their impact on ecosystems and waterways.
Low Toxicity
Sophorolipids exhibit low toxicity compared to many synthetic surfactants. This characteristic enhances their safety profile for both human health and environmental applications, reducing potential risks to workers and end-users.
Versatile Surface Activity
Bio-based emulsifiers, like sophorolipid biosurfactants, possess versatile surface-active properties, allowing them to function effectively in a wide range of formulations. These bio-based emulsifiers can reduce interfacial tension, stabilize emulsions and enhance dispersion and solubilization of hydrophobic compounds.
Improved Stability
Sophorolipids contribute to the stability of emulsions and dispersions by forming a protective layer around dispersed particles or droplets. This helps prevent coalescence and sedimentation, leading to more stable and uniform formulations.
Enhanced Performance
In addition to their surface-active properties, sophorolipid bio-based emulsifiers can offer performance benefits such as improved wetting, foaming and detergency. These attributes make them valuable additives in formulations for cleaning products, personal care items, paints, coatings and agricultural products.
Regulatory Compliance
Sophorolipid biosurfactants are generally recognized as safe (GRAS) by regulatory authorities such as the U.S. Food and Drug Administration (FDA), making them suitable for use in various consumer and industrial applications. They also align with regulatory trends favoring bio-based and environmentally friendly ingredients.
Overall, sophorolipid biosurfactants represent a sustainable and effective solution for formulators seeking bio-based emulsifiers with excellent performance and safety characteristics. Their versatile functionality and eco-friendly profile make them valuable ingredients in the transition towards greener and more sustainable formulations across industries.
Case Study: Advancing Sustainable I&I Cleaning Formulations
Introduction
In the industrial and institutional (I&I) cleaning sector, the quest for effective, environmentally friendly cleaning solutions is paramount. Traditional cleaning formulations often rely on harsh chemical and solvent emulsifiers, posing risks to human health and the environment.
However, the integration of bio-based emulsifiers, specifically sophorolipid biosurfactants, offers a sustainable alternative. The bio-based emulsifiers play a pivotal role in formulating cleaning solutions, enabling the dispersion of hydrophobic and hydrophilic components to enhance cleaning efficacy.
This case study explores the application of sophorolipid biosurfactants as bio-based emulsifiers in an I&I cleaning company and their transformative impact on cleaning effectiveness and sustainability.
Findings
A leading provider of environmentally friendly I&I cleaning products faced challenges in formulating high-performance cleaners that could effectively remove tough soils and stains while minimizing environmental impact.
Traditional formulations struggled to achieve the desired cleaning efficacy without resorting to harsh chemicals and solvents, limiting their sustainability and appeal to environmentally conscious consumers.
To address these challenges, the cleaning solutions provider embarked on a reformulation challenge aimed at integrating sophorolipid biosurfactants as bio-based emulsifiers into their cleaning formulations.
Sophorolipids, derived from microbial fermentation of renewable feedstocks, offer a sustainable and biodegradable alternative to conventional surfactants. These bio-based emulsifiers possess excellent surface-active properties, enabling them to disperse and solubilize oils, greases and other hydrophobic compounds in aqueous solutions.
One key application of sophorolipid biosurfactants as bio-based emulsifiers was in the formulation of industrial degreasers and heavy-duty cleaners. By integrating bio-based emulsifiers into the formulations, the cleaning solutions provider achieved remarkable improvements in soil removal and emulsification of oily residues.
The sophorolipid biosurfactants effectively dispersed the oil and grease particles, allowing for their easy removal from surfaces without the need for harsh chemicals or abrasive scrubbing. This resulted in cleaner surfaces, reduced cleaning times and enhanced overall cleaning efficiency.
Furthermore, sophorolipid biosurfactants played a crucial role in developing multipurpose cleaners for institutional use. These cleaners needed to effectively remove a wide range of soils and stains from various surfaces while being gentle on surfaces and safe for users.
Sophorolipids facilitated the dispersion and solubilization of soils and stains in water, ensuring thorough cleaning without compromising on sustainability or performance.
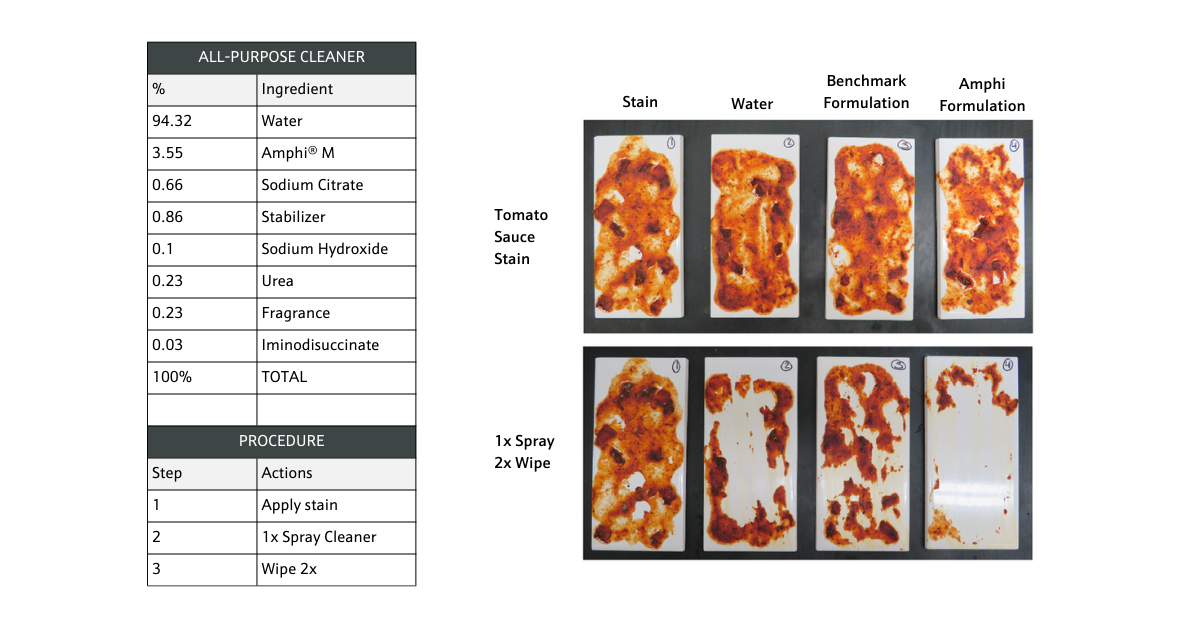
Figure 2. All-purpose cleaner wipe test comparing benchmark with Amphi M formulation.
Moreover, the integration of sophorolipid biosurfactants as bio-based emulsifiers into the cleaning formulations led to improved environmental sustainability.
By reducing the reliance on toxic chemicals and solvents, the cleaning solutions provider minimized the environmental impact of their products, making them safer for use in commercial and institutional settings.
Additionally, the efficient cleaning action of sophorolipid biosurfactants resulted in reduced water consumption and wastewater generation, further contributing to sustainability efforts.
Conclusion
The case study underscores the transformative impact of sophorolipid biosurfactants as bio-based emulsifiers in advancing industrial and institutional cleaning processes.
By harnessing the dispersion-enhancing properties of these bio-based emulsifiers, the cleaning solutions provider achieved superior cleaning performance, reduced environmental impact and enhanced sustainability across their product portfolio.
Sophorolipid biosurfactants emerge as essential additives in I&I cleaning formulations, offering a sustainable solution to meet the evolving needs of the cleaning industry while promoting environmental stewardship.
Formulating a Sustainable Future with Bio-Based Emulsifiers
The clash of the colloids between emulsifiers and dispersants represents a dynamic interplay of scientific principles driving innovation across industries.
Bio-based emulsifiers, exemplified by sophorolipid biosurfactants, are at the forefront of this revolution, offering sustainable solutions that redefine formulation possibilities.
From the multifaceted role of emulsifiers in creating stable emulsions to the crucial function of dispersants in particle dispersion, the article has provided insights into the intricate world of colloidal chemistry.
By harnessing the power of bio-based emulsifiers, formulators can achieve superior performance while minimizing environmental impact and meeting the demands of eco-conscious consumers.
As the need for sustainable solutions continues to grow, bio-based emulsifiers emerge as key drivers of change, facilitating the transition towards greener and more sustainable formulations. By exploring the science behind emulsifiers and dispersants, we can unlock new possibilities for innovation and shape a more sustainable future for generations to come.
Ready to Innovate Your Formulations?
Explore our Technical Library for a wealth of resources on sustainable formulations using bio-based emulsifiers like sophorolipid biosurfactants. Access SDSs, one-pagers, and more now!
Dispersing Agents: The Role Of Sophorolipid Biosurfactants In Greening Particle Dispersion
READ MORE: What Are Dispersants?
Dispersants are substances or chemicals that are used to improve the dispersion or uniform distribution of particles in a medium, typically a liquid. The primary goal of dispersants is to prevent agglomeration or clumping of particles, enabling them to remain stably dispersed throughout the medium. Dispersants find applications in various industries, including paints, inks, coatings, textile dyeing and more. [Read the full article here]