Putting the FACT in Surfactants: Technical Details
Surfactant ingredients play a pivotal role in various sectors, spanning from industrial processes to personal care products and household cleaners.
Whether bio-based or synthetic, surfactants are multifunctional. They can serve purposes ranging from getting those stubborn stains up, to washing hair or cleaning countertops. But what are surfactants and what is their purpose within product formulations?
This article is the second in the “Putting the FACT in SurFACTants” series, which will include:
- Surfactant Overview
- Technical Details
- Classifications
- Applications
What Is A Surfactant?
At the heart of surfactants lies their primary function as surface-active agents. This characteristic is succinctly encapsulated in the term “surfactant,” derived from its role in chemistry.
Surfactant Ingredients As Surface Active Agents
Surface-active agents possess a remarkable ability to diminish the surface or interfacial tension between two liquids, such as water.
For instance, water molecules exhibit strong bonds with each other, resulting in notable surface tension, which allows water bugs to glide effortlessly across a pond’s surface.
Surfactants intervene by disrupting the hydrogen bonds between water molecules, thereby reducing interfacial tension and facilitating the creation of stable, finely dispersed mixtures.
Moreover, surfactants enhance the wettability of liquids, enabling them to spread and penetrate surfaces effectively. This property is particularly advantageous in products like detergents, where improved penetration and soil dissolution are paramount for enhanced efficacy.
The key to understanding surfactants is to look at their primary function, which forms the basis for their name. The word “surfactant” is an abbreviation of its role in chemistry as a surface active agent.
Wet⋅ta⋅bil⋅i⋅ty
(noun)
When referring to the absorption of solids into a liquid such as water, wettability refers to the time (in seconds) that it takes for a powder to penetrate the still surface of the water (or liquid) and absorb it.
Surfactants As Surface Active Agents
Surface active agents have the unique ability to decrease the surface or interfacial tension between two liquids, such as water.
For example, water molecules form strong bonds with each other creating impressive surface tension. These bonds and resulting tension are the reason why water bugs can “skate” across the surface of a pond. Surfactants decrease the interfacial tension of water by interfering with the hydrogen bond between the water molecules.
Surfactants Improve Wettability
By reducing the surface tension of the water (or other liquids), surfactants help foster a stable, finely distributed mixture.
The Molecular Dynamics Of Surfactant Ingredients
Surfactant ingredients boast a molecular structure comprising hydrophilic (water-loving) heads and hydrophobic (water-fearing) tails. Upon encountering a liquid surface, the hydrophilic heads orient themselves towards the liquid, while the hydrophobic tails extend outward.
How Do Surfactants Break Interfacial Bonds?
As surfactant molecules saturate the liquid surface, they congregate to form micelles, specialized structures that disrupt interfacial bonds. These micelles, characterized by their varying sizes, possess the ability to penetrate surface imperfections, such as cracks and pores, thereby enhancing their cleaning and emulsifying capabilities.
Surfactants Reduce Surface Tension
During this process, new bonds are formed. The molecular force between a surfactant molecule and a water molecule is lower than the force between two water molecules. This reduces surface tension and allows for deep penetration and improved performance in product formulations.
Formulators leverage the unique molecular properties of these surface active agents, or surfactant ingredients, to improve the performance of products such as lotions, detergents and degreasers.
The Use Of Chemical Surfactant Ingredients
There are many different classes and families of surfactants, each with their own unique applications.
Chemical Surfactants
Synthetic or chemical surfactants have long dominated the market, boasting high performance and foaming capabilities. However, they often raise environmental concerns due to their reliance on nonrenewable resources and potential adverse effects on skin and the ecosystem.
Chemical surfactants encompass a wide range of compounds that are widely used in various industries. Here are some examples of chemical surfactants:
Sodium Lauryl Sulfate (SLS):
- SLS is one of the most commonly used surfactants in personal care products such as shampoos, body washes and toothpaste.
- It is prized for its ability to create a rich lather and effectively remove dirt and oil from the skin and hair.
- However, SLS can be harsh on the skin and may cause irritation, particularly for individuals with sensitive skin.
Cocamidopropyl Betaine (CAPB):
- CAPB is a surfactant derived from coconut oil and is widely used in shampoos, conditioners and other personal care products.
- It serves as a co-surfactant, helping to increase the foaming properties of formulations and improving their overall performance.
- CAPB is known for its mildness and is often used in products marketed as “gentle” or “sensitive skin-friendly.”
- Although CAPB is generally considered to be gentler than some other surfactants, it is not entirely free from the risk of irritation or allergic contact dermatitis.
- For individuals prone to skin sensitivities, especially those with eczema or other dermatological conditions, products containing CAPB may exacerbate existing issues or cause new reactions.
Ethoxylated Surfactants:
- Ethoxylated surfactants are a class of surfactants produced through ethoxylation, a chemical process that involves reacting ethylene oxide with fatty acids or alcohols.
- Examples include various types of ethoxylated alcohols, fatty acids, and amines.
- These surfactants are used in a wide range of applications, including household cleaners, industrial formulations, and personal care products.
- Unfortunately, 1,4-dioxane, a forever chemical and possible carcinogen, is a byproduct of the ethoxylation process.
Alkylbenzene Sulfonates:
- Alkylbenzene sulfonates are synthetic surfactants commonly used in laundry detergents and dishwashing liquids.
- They exhibit excellent cleaning properties and are effective at removing grease, oil and other stains from fabrics and dishes.
- However, they may be less biodegradable than some other surfactants, posing environmental concerns in certain formulations.
Quaternary Ammonium Compounds (Quats):
- Quats are a class of surfactants known for their antimicrobial properties and are commonly used as disinfectants and sanitizers.
- They are effective against a wide range of microorganisms, including bacteria, viruses and fungi.
- Quats are frequently used in healthcare settings, food processing facilities and household cleaning products.
- While quats are effective antimicrobial agents commonly used in disinfectants, sanitizers and cleaning products, they can persist in the environment and may have adverse effects on aquatic ecosystems.
These examples represent just a few of the many chemical surfactants used in various industries. Each surfactant has its own unique properties and applications, making them essential components in countless products around the world.
These common surfactants rely on nonrenewable resources such as petrochemicals. While some of them boast high performance and great foaming capabilities, there are downsides. Chemical surfactants may cause skin irritation, they are not biodegradable and they can negatively impact the environment.
Chemical surfactants also have simple molecular structures, making them not as multifunctional within product formulations.

Palm Oil-Based Surfactants
Examples of palm oil-based surfactants include:
Sodium Laureth Sulfate (SLES):
- SLES is a widely used surfactant in personal care and household cleaning products.
- It is derived from ethoxylated lauryl alcohol, which can be sourced from palm oil.
- SLES is valued for its foaming and cleansing properties and is commonly found in shampoos, body washes and liquid soaps.
- The production of SLES from palm oil can contribute to deforestation, habitat destruction and biodiversity loss, especially if the palm oil is not sustainably sourced.
Sodium Lauryl Sulfate (SLS):
- SLS is another common surfactant derived from lauryl alcohol, which can be derived from palm oil.
- Like SLES, SLS is prized for its ability to create lather and effectively remove dirt and oil from surfaces.
- It is used in a wide range of personal care and household cleaning products.
- SLS has the potential to cause skin irritation, especially in individuals with sensitive skin or certain skin conditions like eczema.
Ethoxylated Alcohols:
- Ethoxylated alcohols are a group of surfactants produced through the ethoxylation of fatty alcohols, which can be derived from palm oil.
- These surfactants are used in various applications, including laundry detergents, dishwashing liquids and industrial cleaners.
- Ethoxylated alcohols are valued for their cleaning and emulsifying properties.
- Ethoxylated alcohols can pose risks to aquatic environments due to their potential to bioaccumulate and persist in waterways.
Alkyl Polyglucosides (APGs):
- APGs are nonionic surfactants synthesized from natural sugars and fatty alcohols, which can include palm-derived ingredients.
- They are considered to be environmentally friendly and biodegradable, making them attractive for use in green cleaning products.
- APGs are used in a range of applications, including personal care products, household cleaners and industrial formulations.
- APGs can be more expensive to produce compared to conventional surfactants like SLES and SLS.
These are just a few examples of palm oil-based surfactants commonly used in consumer products.
While palm oil-derived ingredients offer functional benefits and versatility in formulations, concerns about the environmental impact of palm oil cultivation, including deforestation and habitat destruction, have led to increased interest in sustainable alternatives.
Bio-Based Surfactants
The downsides of synthetic chemical surfactant ingredients have increased the demand for bio-based and natural surfactants. These alternative options include:
Alkyl Polyglucosides (APGs):
- APGs are nonionic surfactants derived from natural sugars (typically glucose) and fatty alcohols. They are produced through a condensation reaction between glucose and fatty alcohols obtained from renewable sources like coconut oil or corn.
- APGs are valued for their biodegradability, low toxicity, and excellent cleaning properties. They are used in a variety of applications, including personal care products, household cleaners, and industrial formulations.
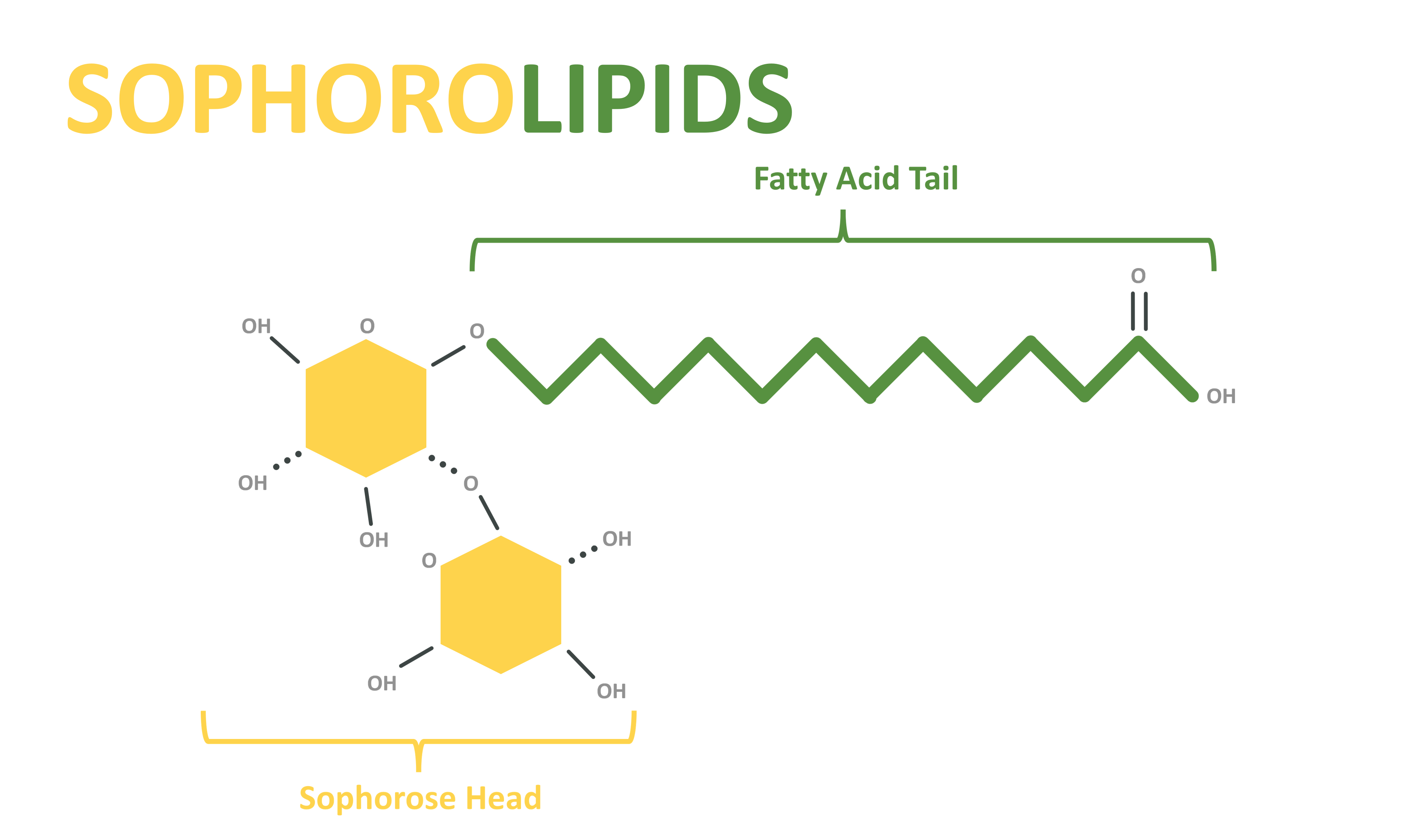
Sophorolipids:
- Sophorolipids are glycolipid surfactants produced by yeast species, particularly Candida bombicola. They are composed of sophorose sugar molecules and fatty acids.
- Sophorolipids are known for their exceptional surface tension reduction, emulsification, and antimicrobial properties. They are used in various industries, including cosmetics, pharmaceuticals, and environmental remediation.
Mannosylerythritol Lipids (MELs):
- MELs are biosurfactants produced by yeast species, such as Candida antarctica. They consist of mannose sugar molecules and fatty acids.
- MELs exhibit surface-active properties and have applications in fields such as cosmetics, food processing, and bioremediation.
Rhamnolipids:
- Rhamnolipids are microbial surfactants produced by various bacterial species, such as Pseudomonas aeruginosa. They are composed of rhamnose sugar molecules and fatty acids.
- Rhamnolipids exhibit excellent surface-active properties, making them suitable for applications such as enhanced oil recovery, bioremediation, and microbial control in agriculture.
Saponins:
- Saponins are naturally occurring surfactant compounds found in various plant species, including soapwort, quillaja, and yucca. They are composed of a hydrophilic sugar moiety and a hydrophobic steroid or triterpenoid backbone.
- Saponins have foaming, emulsifying, and detergent properties, making them useful in applications such as personal care products, food processing, and agricultural formulations.
These examples highlight the diversity of bio-based surfactants available, each with its own unique properties and applications. Bio-based surfactants offer a sustainable alternative to conventional surfactants derived from petrochemicals, contributing to the reduction of environmental impact and promoting the use of renewable resources.
While considered bio-based, APGs get their carbon from plant-based sources, including palm oil. They are also made through carbon-intensive synthetic chemical conversions. Their performance and functionality vary greatly depending on the fatty acid used in their production.
It’s important to note that all biosurfactants are bio-based surfactants, but not all bio-based surfactants are biosurfactants.
Because of these factors, many formulators are turning towards biosurfactants such as sophorolipids, rhamnolipids and MELs.
Biosurfactants: The Natural Surfactant Alternative
In addition to better efficacy, Locus Ingredients’ sophorolipid biosurfactants offer a multitude of sustainability benefits. They have a low carbon footprint and the highest renewable carbon index rating. Each sophorolipid line is USDA certified as 100% biobased and readily biodegradable. The biosurfactants are non-GMO, sulfate free and palm oil free. They have no Proposition 65 issues, 1,4-dioxane or ethylene oxide.
biosurfactants offer a multitude of sustainability benefits. They have a low carbon footprint and the highest renewable carbon index rating. Each sophorolipid line is USDA certified as 100% biobased and readily biodegradable. The biosurfactants are non-GMO, sulfate free and palm oil free. They have no Proposition 65 issues, 1,4-dioxane or ethylene oxide.
The Ultimate Q&A Surfactant Guide
1. What are surfactants, and why are they important?
Answer: Surfactants, short for surface-active agents, are compounds with unique properties that reduce surface tension and facilitate the dispersion of substances in liquid solutions. They play a pivotal role in various industries, enhancing the effectiveness of cleaning, wetting and emulsification processes.
2. What are the benefits of using bio-based surfactants?
Answer: Bio-based surfactants offer several advantages over traditional, petroleum-derived counterparts. They are derived from renewable resources, such as plant oils or microbial fermentation, reducing reliance on fossil fuels. Additionally, bio-based surfactants tend to have lower environmental impact and may offer comparable or superior performance in formulations.
3. What are biosurfactants, and why are they gaining attention?
Answer: Biosurfactants are a specific category of bio-based surfactants produced through microbial fermentation processes. They boast complex molecular structures and exhibit excellent surface-active properties. Biosurfactants are gaining attention for their potential to offer high performance while minimizing environmental impact, making them ideal candidates for sustainable formulations.
4. Can you provide examples of bio-based surfactants?
Answer: Some examples of bio-based surfactants include alkyl polyglucosides (APGs), sophorolipids, rhamnolipids and mannosylerythritol Lipids (MELs). These surfactants are derived from renewable resources and are increasingly being used in various industries for their eco-friendly characteristics.
5. How do surfactants contribute to sustainability in product formulations?
Answer: By choosing bio-based and biosurfactant alternatives, companies can reduce their ecological footprint while maintaining product performance. These surfactants are biodegradable, non-toxic and often derived from sustainable sources, aligning with principles of environmental stewardship and corporate responsibility.
6. What are the key properties of surfactants that make them versatile in various applications?
Answer: Surfactants possess hydrophilic (water-attracting) and hydrophobic (water-repelling) properties due to their molecular structure. This dual nature allows them to interact with both water and oil, making them effective in processes such as emulsification, wetting and foaming.
7. How do bio-based surfactants compare to traditional surfactants in terms of performance and effectiveness?
Answer: Bio-based surfactants have made significant advancements in recent years, offering performance comparable to or even surpassing that of traditional surfactants. Advances in formulation technology and manufacturing processes have enabled bio-based surfactants to meet or exceed industry standards for efficacy and functionality.
8. What are some of the challenges associated with the widespread adoption of bio-based surfactants?
Answer: Despite their many benefits, bio-based surfactants may face challenges related to scalability, cost-effectiveness and market acceptance. Scaling up production processes and ensuring consistent quality at competitive prices can be hurdles for manufacturers. Additionally, consumer education and awareness initiatives may be needed to promote the value proposition of bio-based surfactants compared to traditional alternatives.
9. How do biosurfactants contribute to sustainability beyond their environmental benefits?
Answer: Biosurfactants offer a holistic approach to sustainability by not only reducing environmental impact but also supporting social and economic sustainability. Their production often involves utilizing waste streams or agricultural byproducts, contributing to resource efficiency and circular economy principles. Furthermore, biosurfactant production can create opportunities for rural communities and promote local economic development in regions where raw materials are sourced.
10. What role do regulatory standards and certifications play in the adoption of bio-based surfactants?
Answer: Regulatory standards and certifications, such as USDA BioPreferred and EcoLogo, play a crucial role in validating the environmental claims of bio-based surfactants. These certifications provide assurance to consumers and businesses regarding the sustainability credentials of products. Compliance with regulatory requirements also helps manufacturers demonstrate their commitment to environmental responsibility and transparency.
READ MORE: Putting The FACT In SurFACTants: Part 3 Classifications
Surfactants are one of the most versatile ingredients for use in almost every industry. It is already known that these surface-active agents serve a multitude of purposes from homecare, personal care, and even industrial applications. They make strong detergents, wetting agents, emulsifiers, and more.
But did you know there are actually four different types of classifications for surfactants?
Four Surfactant Classifications
Every surfactant is composed of two separate regions—the hydrophobic tail and the hydrophilic head. It is the hydrophilic head that separates one surfactant from the next. There are four types of surfactant classifications [Read the full article here]




